Metals represent a class of chemical elements defined by distinctive characteristics. They are recognized for their metallic luster, high electrical conductivity, malleability, and ductility.
hat sets metals apart is their ability to readily relinquish electrons, forming positively charged cations within their crystalline atomic structure. These cations are held together by metallic bonds, where the outermost electrons of metal atoms become delocalized, creating a “sea of electrons.” This unique electron mobility imparts key attributes to metals, such as excellent electrical and thermal conductivity.
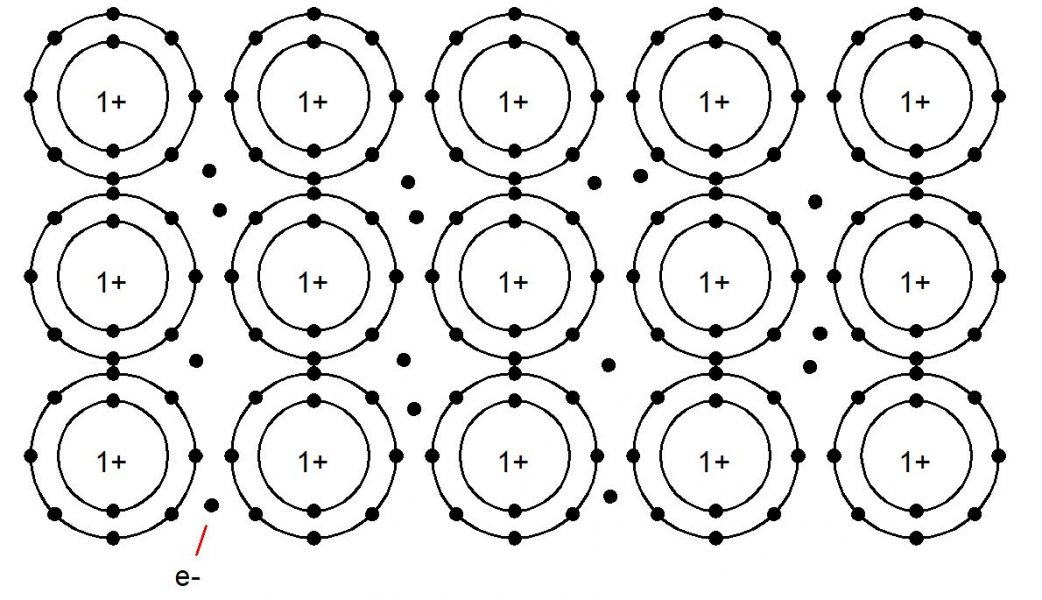
Metals are typically solid at room temperature and exhibit a diverse range of physical and mechanical properties, making them indispensable materials across multiple industries. Their fundamental role in technology and society stems from this distinctive atomic structure and behavior.
