Polyethylene terephthalate (PET) is a thermoplastic polyester formed by the condensation polymerization of ethylene glycol and terephthalic acid. Its repeating unit contains an aromatic ring linked by ester functional groups, resulting in a linear polymer chain with relatively high stiffness compared to many polyolefins. The presence of the benzene ring in the backbone restricts chain rotation, contributing to higher strength, improved thermal stability, and greater resistance to creep under load. As with other thermoplastics, PET can be melted and reshaped, but its chemical structure leads to higher processing temperatures than those required for polyethylene or polypropylene.
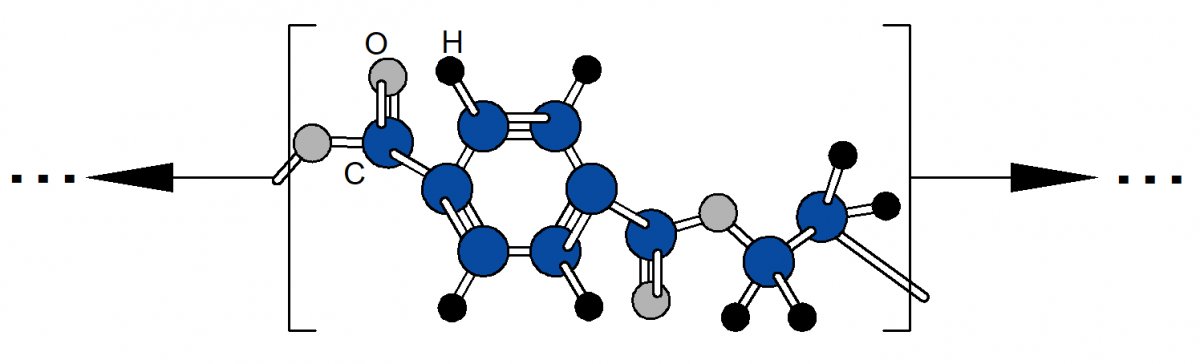
The material is typically semi-crystalline, with properties strongly influenced by processing history and cooling rate. Rapid cooling tends to produce a more amorphous structure, yielding transparent and tough products, while slower cooling or controlled reheating can increase crystallinity and improve stiffness, chemical resistance, and dimensional stability. PET exhibits good barrier properties to gases such as oxygen and carbon dioxide, along with low moisture absorption, making it well suited for applications where containment and product preservation are important.
In manufacturing, PET is widely processed by injection molding and stretch blow molding, particularly for beverage containers and packaging films. The ability to orient the polymer chains during stretching enhances strength and impact resistance, illustrating the strong relationship between molecular alignment and mechanical performance. In addition to packaging, PET fibers are used in textiles and reinforcement applications due to their high tensile strength and durability. Its recyclability and widespread use have also made PET a central material in discussions of polymer recovery and sustainable manufacturing practices.
